Introduction
Antibody discovery often begins with strong biological activity, but early success does not guarantee clinical viability. One of the most common barriers to translation is immunogenicity. According to analyses referenced by the National Institutes of Health, immune responses against non-human antibody sequences remain a major cause of therapeutic failure during development.
This is where an antibody humanization service becomes critical. By modifying antibody sequences to more closely resemble human antibodies while preserving binding specificity, humanization bridges the gap between discovery and clinical application. It is not simply an optimization step. It is often a prerequisite for moving forward.
What Is an Antibody Humanization Service?
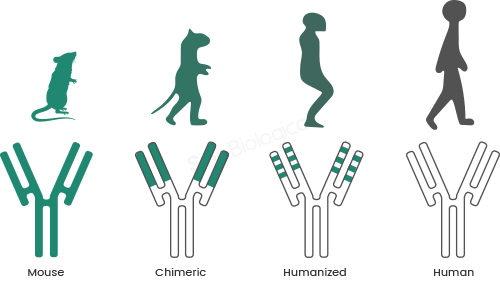
An antibody humanization service focuses on reducing the immunogenic potential of antibodies originally derived from non-human sources, such as murine or other animal models. The goal is to retain antigen-binding regions while replacing framework sequences with human equivalents.
Most humanization strategies rely on complementarity-determining region (CDR) grafting, where key binding loops are transferred onto human antibody frameworks. Advanced services go further, incorporating back-mutations, structural modeling, and sequence analysis to preserve affinity and stability.
The outcome is a humanized antibody that maintains functional performance while being better tolerated by the human immune system.
Real-World Impact of an Antibody Humanization Service
The importance of antibody humanization is well documented across therapeutic areas. Early murine antibodies demonstrated strong efficacy but frequently triggered anti-drug antibody responses in patients, limiting their usefulness.
Research indexed in PubMed shows that humanized antibodies exhibit significantly reduced immunogenicity compared to their fully murine predecessors. This improvement often translates into longer half-life, improved dosing profiles, and more consistent clinical responses.
In practical terms, an antibody humanization service enables promising antibody candidates to remain viable as they progress into preclinical and clinical studies. Without this step, many otherwise effective antibodies would be abandoned early.
Why an Antibody Humanization Service Matters for the Future
The antibody landscape is becoming increasingly competitive. Many targets are shared across multiple development programs, and differentiation often depends on safety, durability, and manufacturability rather than simple target engagement.
Humanization plays a key role in this differentiation. Antibodies that are well tolerated and structurally stable are easier to scale, formulate, and regulate. As therapeutic formats diversify, including bispecific antibodies and antibody-drug conjugates, sequence optimization becomes even more important.
There is also growing regulatory emphasis on immunogenicity risk assessment. Agencies increasingly expect early mitigation strategies rather than reactive fixes. Antibody humanization services align with this expectation by addressing risk at the molecular design stage.
Scientific Considerations in Antibody Humanization
Humanization is not a purely mechanical process. Poorly executed CDR grafting can reduce affinity, alter specificity, or compromise stability. Effective antibody humanization services integrate sequence analysis, structural modeling, and functional validation to avoid these pitfalls.
Publications in Nature highlight how subtle framework interactions influence antigen binding. Preserving these interactions requires careful design and iterative testing.
As a result, humanization is best approached as a data-driven optimization process rather than a one-time modification.
Benefits for Researchers and Development Teams
For researchers, antibody humanization services provide confidence that lead candidates are suitable for downstream development. Functional antibodies can be tested in more clinically relevant models without confounding immune reactions.
For development teams, early humanization reduces late-stage redesign. Programs move forward with clearer regulatory pathways and more predictable manufacturing behavior.
For healthcare systems, successful humanization supports the development of safer and more effective antibody-based therapies, ultimately benefiting patient outcomes.
Regulatory and Translational Relevance
Global health and regulatory organizations, including the World Health Organization, emphasize the importance of safety and predictability in biologic development. Antibody humanization directly supports these goals by reducing immune-mediated risks.
As antibody therapies expand into chronic and preventive indications, long-term tolerability becomes increasingly important. Humanization helps ensure antibodies can be administered repeatedly without triggering adverse immune responses.
Conclusion
Discovery alone does not deliver therapies. Translation depends on molecular refinement and risk reduction. An antibody humanization service enables antibody candidates to retain their biological activity while meeting the safety expectations required for clinical development.
As therapeutic antibody pipelines continue to grow, antibody humanization service solutions will remain a cornerstone of responsible and successful antibody optimization.